14+ li orbital diagram
Orbital diagram of Sodium Na 12. Lithium Oxygen Fluorine Bromine Silicon Zinc electrons Electron Configuration 15201 Orbital Diagram 2p 14 1 36.

Lithium Orbital Diagram Electron Configuration And Valence Electrons
Next we go to lithium with 3 electrons.

. When we talk about the orbital diagram we first need to understand what exactly it means. Orbital diagram of Lithium Li 4. Which reaction below represents the electron affinity of Li.
The Silicon orbital diagram contains 2 electrons in 1s orbital 2 electrons in the 2s orbital the six electrons in the 2p orbital the two electrons in the 3s orbital and the remaining two electrons. Um was a toupee sub shell. One of the B-F bonds lies along the x-axis as shown in.
It has a melting point of 1414c and a boiling point of 3265c. It is located in Group 14 as a metalloid solid at room temperature it is relatively light weight and strong. A Lig e Lig.
If more than one molecular orbital of the same energy is available the electrons enter them singly and adopt parallel spins Hunds Rule. We already know that the p-subshell has three orbitals. Helium has 2 electrons and both electrons go into the 1s orbital and the electron configuration is 1s2.
The highest energy orbital is the top orbital. Choose the valence orbital diagram that represents the ground state of Sr2 look at question 15 page 2 E. Orbital diagram of Boron B 6.
Therefore during exams the student can. The highest occupied molecular orbital is the third orbital is the third all. How many users might wonder what exactly will be the problem with that.
Orbital diagram of Beryllium Be 5. Check all that apply. The orbitals are p x p y and p z and each orbital can.
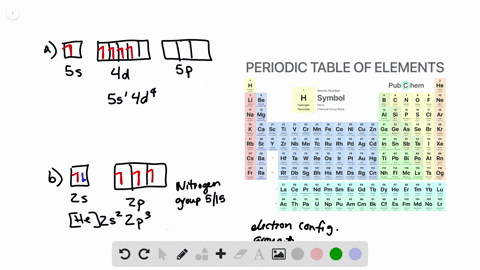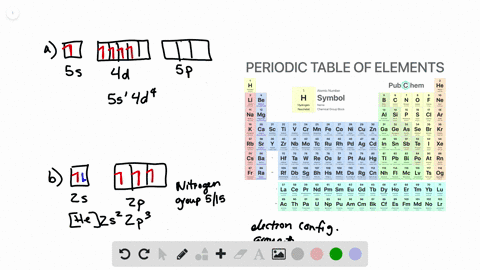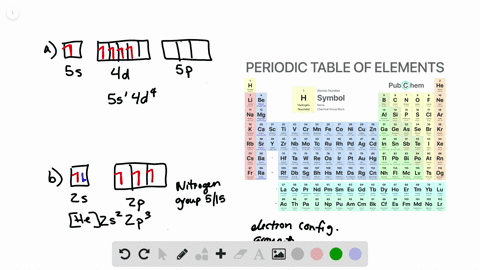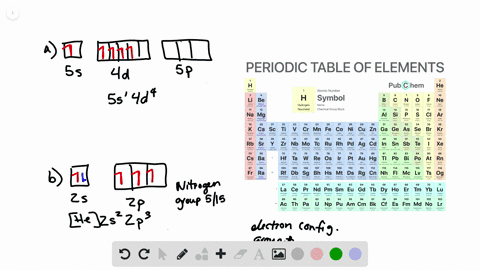
What is the Orbital Diagram For Nitrogen. Electrons always occupy the lowest energy orbitals available a single orbital can hold a maximum of two electrons electrons occupying the same orbital must have the. 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 6 4d 10 5s 2 5p 6 4f 14 5d 10 6s 2 6p 6 5f 14 6d 10 7s 2 7p 1.
When the beryllium atom is excited then the beryllium atom absorbs energy. So the highest energy or bitten will have A total of five notes auction D. Electron configuration of Flerovium Fl.
7 14 in the. Orbital diagrams Orbital box diagrams of all elements are mentioned in the chart given below. 2 8 18 32 32 18 3.
Orbital diagram of Carbon C 7. The ground-state electron configuration of beryllium is 1s 2 2s 2. So heres the electron configuration of nitrogen now without adding any more orbitals.
As a result an electron in the 2s orbital jumps. The ground state electron configuration of silicon is 1s 2 2s 2 2p 6 3s 2 3p 2. That means the highest possible orbital cookies to P.
The orbital diagram of arsenic can be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p3. Carbon Electron Configuration is an element of the periodic table and its atomic number is 6. Download Silicon as a.
The electron configuration is 1s1.
Molecular Orbital Theory Mot Chemistry Study Material Emedicalprep Com Emedicalprep
Molecular Orbital Diagram Wikipedia

Lithium Li Orbital Diagram Electron Configuration And Valence Electrons In 2022 Electron Configuration Electrons Configuration

Structural Diversities In Heterometallic Mn Ca Cluster Chemistry From The Use Of Salicylhydroxamic Acid Mniii4ca2 Mnii Iii6ca2 Mniii Iv8ca And Mniii8ca2 Complexes With Relevance To Both High And Low Valent States Of The Oxygen Evolving
Chem4kids Com Lithium Orbital And Bonding Info
Chapter 8 Electron Configuration And Chemical Periodicity Video Solutions Chemistry The Molecular Nature Of Matter And Change Numerade

How To Draw Molecular Orbital Diagram Of Li2 Li 2 Li2

Lithium Orbital Diagram Electron Configuration And Valence Electrons

Diagram Of Orbital Electron Energies For The C ϩ He 6 ϩ Download Scientific Diagram

Lithium Electron Configuration Youtube

In Molecular Orbital Theory Why Do We Follow A Certain Electronic Configuration Upto Nitrogen And After That Electronic Configuration Is Changed Why Quora
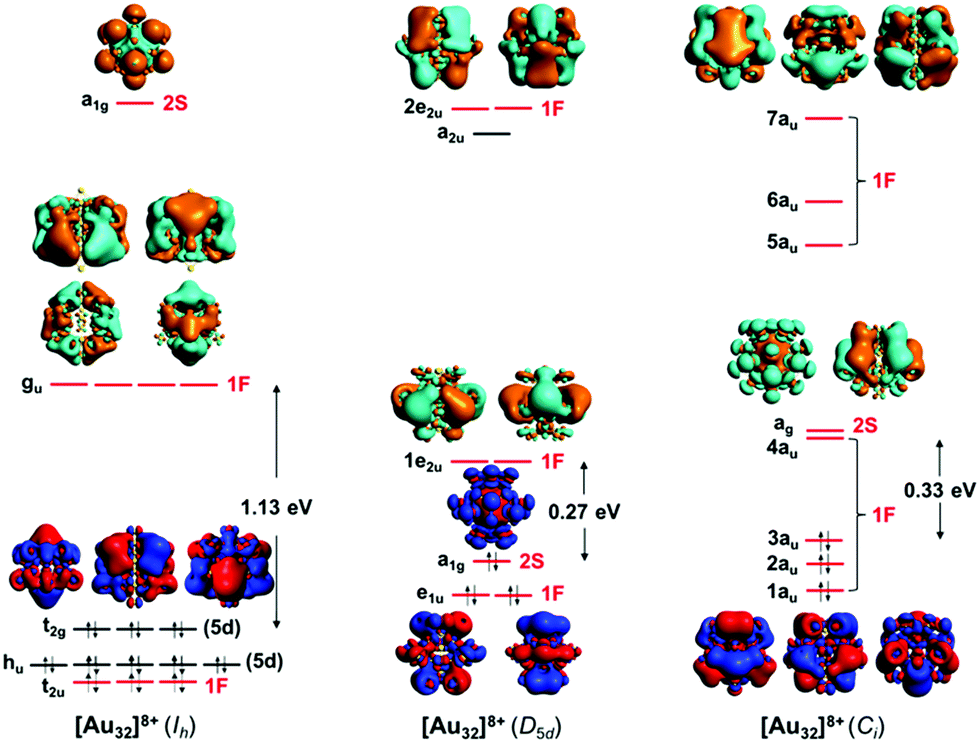
Electron Count And Electronic Structure Of Bare Icosahedral Au 32 And Au 33 Ionic Nanoclusters And Ligated Derivatives Stable Models With Intermediat Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 D0cp03735d
Chem4kids Com Lithium Orbital And Bonding Info

The Orbital Diagram Which Violates The Aufbau Principle Is
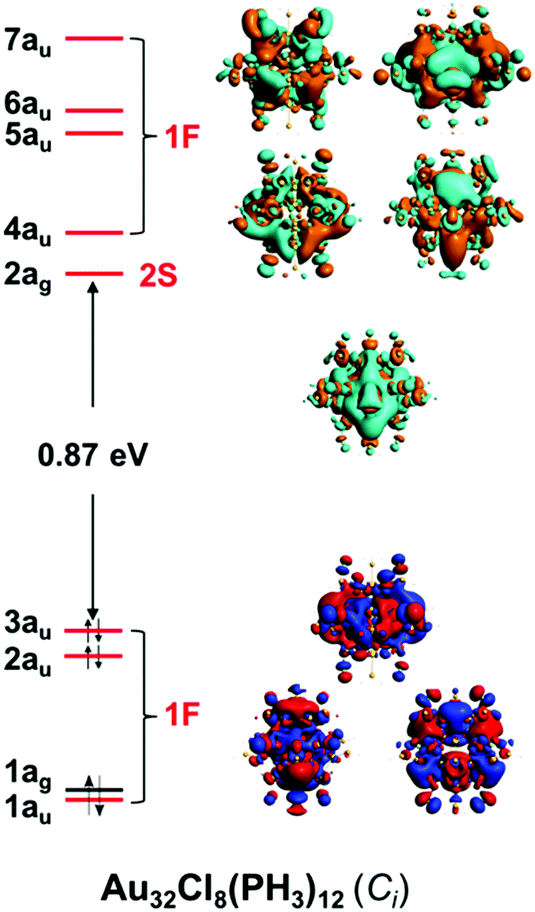
Electron Count And Electronic Structure Of Bare Icosahedral Au 32 And Au 33 Ionic Nanoclusters And Ligated Derivatives Stable Models With Intermediat Physical Chemistry Chemical Physics Rsc Publishing Doi 10 1039 D0cp03735d
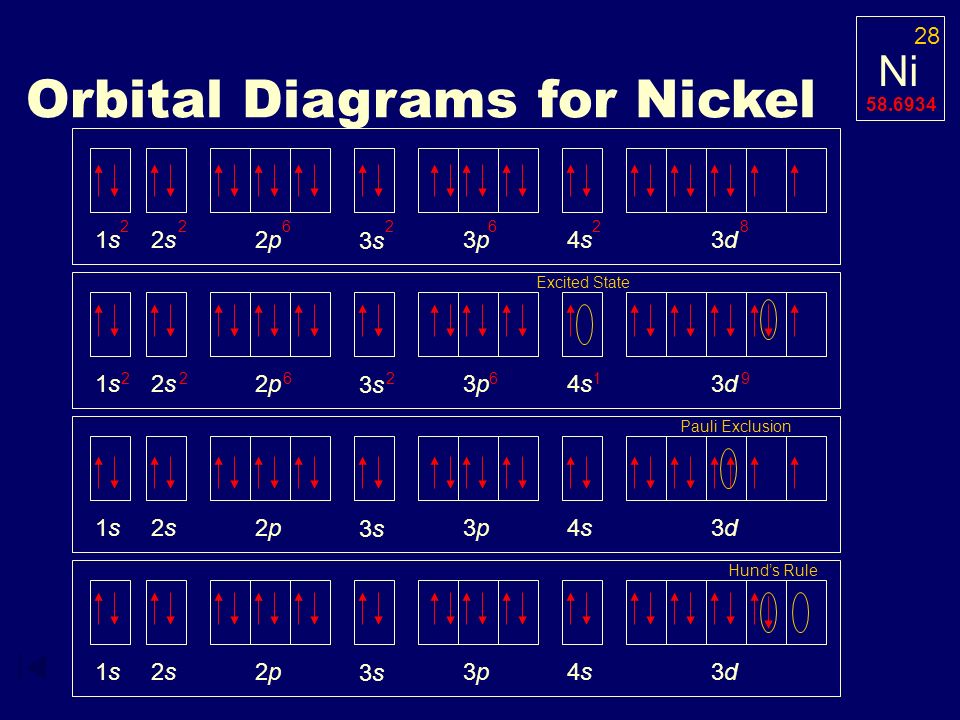
Electron Configuration Ppt Video Online Download

Electron Configuration For Lithium Li